Montages for tCS
In this page we provide some examples of tCS montages used in the literature for several applications (for tDCS mostly), and we provide related multichannel solutions using Starstim (Stimulation device). Readers are encouraged to search the literature for other options (but see the References for our White Papers on these subjects).
To learn more, please read our review on tCS models and technologies<ref>Giulio Ruffini, Fabrice Wendling, Isabelle Merlet, Behnam Molaee-Ardekani, Abeye Mekonnen, Ricardo Salvador, Aureli Soria-Frisch, Carles Grau, Stephen Dunne, and Pedro C. Miranda, Transcranial Current Brain Stimulation (tCS): Models and Technologies, IEEE TRANSACTIONS ON NEURAL SYSTEMS AND REHABILITATION ENGINEERING, VOL. 21, NO. 3, MAY 2013 </ref> and see our section on StimWeaver.
We recall here the logic regarding anodal versus cathodal stimulation in tDCS (and more generally, tACS and tRNS, with their time dependent modulation). Anodal stimulation over an area produces electric fields directed generally inward into the brain in the subjacent cortex. The direction of the electric field with respect to the orientation of the neuron is a significant parameter in the alteration of the trans-membrane potential, especially of elongated neurons such as pyramidal cells. For this reason we may loosely say that anodal stimulation is excitatory, since long cortical neurons are generally aligned perpendicular to the cortical surface, etc. The opposite applies to cathodal stimulation. However, these are approximate statements. The geometry of the cortical surface is complex, as are the generated electric fields. For this reason, biophysical modeling of electric fields an their interactions with neurons is an important tool to carefully define montages. If interested in the topic, see this paper on biophysical modeling <ref>Giulio Ruffini, Fabrice Wendling, Isabelle Merlet, Behnam Molaee-Ardekani, Abeye Mekonnen, Ricardo Salvador, Aureli Soria-Frisch, Carles Grau, Stephen Dunne, and Pedro C. Miranda, Transcranial Current Brain Stimulation (tCS): Models and Technologies, IEEE TRANSACTIONS ON NEURAL SYSTEMS AND REHABILITATION ENGINEERING, VOL. 21, NO. 3, MAY 2013 </ref> and | Miranda et al. 2013 <ref name="Miranda2013a"> Miranda, P.C., Mekonnen, A., Salvador, R., Ruffini, G., 2013. The electric field in the cortex during transcranial current stimulation. Neuroimage 70, 45–58. </ref> on the electric field generated by focal tDCS.
A recent paper discusses the optimization of tCS montages starting from well defined cortical target maps <ref> Giulio Ruffini, Michael D. Fox, Oscar Ripolles, Pedro Cavaleiro Miranda, Alvaro Pascual-Leone, Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields, available online, Dec 15, Neuroimage (2013) </ref>.
A group of European experts was commissioned by the European Chapter of the International Federation of Clinical Neurophysiology to gather knowledge about the state of the art of the therapeutic use of transcranial direct current stimulation (tDCS) from studies published up until September 2016, regarding pain, Parkinson’s disease, other movement disorders, motor stroke, poststroke aphasia, multiple sclerosis, epilepsy, consciousness disorders, Alzheimer’s disease, tinnitus, depression, schizophrenia, and craving/addiction. The evidence-based analysis included only studies based on repeated tDCS sessions with sham tDCS control procedure:
https://www.clinph-journal.com/article/S1388-2457(16)30634-4/fulltext
Using a multichannel setup offers more focality and versatility than the classical bipolar montages using sponge electrodes. If interested, contact us for our StimWeaver service: we can produce the best multichannel tCS (MtCS) montage for your targeting problem.
Contents
Stroke
Please see our recent White Paper on tCS in Stroke<ref > G. Ruffini, tDCS clinical research - highlights: Stroke, NE White Paper NEWP201302, 2013 </ref>.
A stroke that affects the cerebral cortex may have a wide range of effects depending on the location of the lesion. The clinical strategies for treating stroke typically involve stabilization of the patient, preservation of function in the brain area and adaptation of the patient to diminished function. There are some hints that electrical stimulation of the brain may in itself promote recovery or preservation of brain tissue <ref> Kanzaki S, Stöver T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y., | Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation, J Comp Neurol. 2002 Dec 16;454(3):350-60. </ref>, although to date a relatively small number of published studies have focused on improving specific functions through the use of single or repeated sessions of anodal stimulation.
The main motivation behind the use of non-invasive brain stimulation for stroke recovery is to support relearning of compromised abilities by enhancement of pathologically-reduced cortical excitability and activity, directly by excitability-enhancing brain stimulation of the lesioned area, or indirectly, by reducing excitability of the non-lesioned contralateral hemisphere – since this has inhibitory connections with the lesioned one <ref> Wittenberg GF, Schaechter JD., The neural basis of constraint-induced movement therapy , Curr Opin Neurol. 2009 Dec;22(6):582-8. </ref>. Specifically, the respective excitability enhancements are thought to promote relearning of functions by enhancing learning-related long-term potentiation (LTP) (which is the likely physiological basis of learning and memory formation <ref> Rioult-Pedotti MS, Friedman D, Donoghue JP., Learning-induced LTP in neocortex., Science. 2000 Oct 20;290(5491):533-6 </ref>. ) and via this mechanism promote recovery.
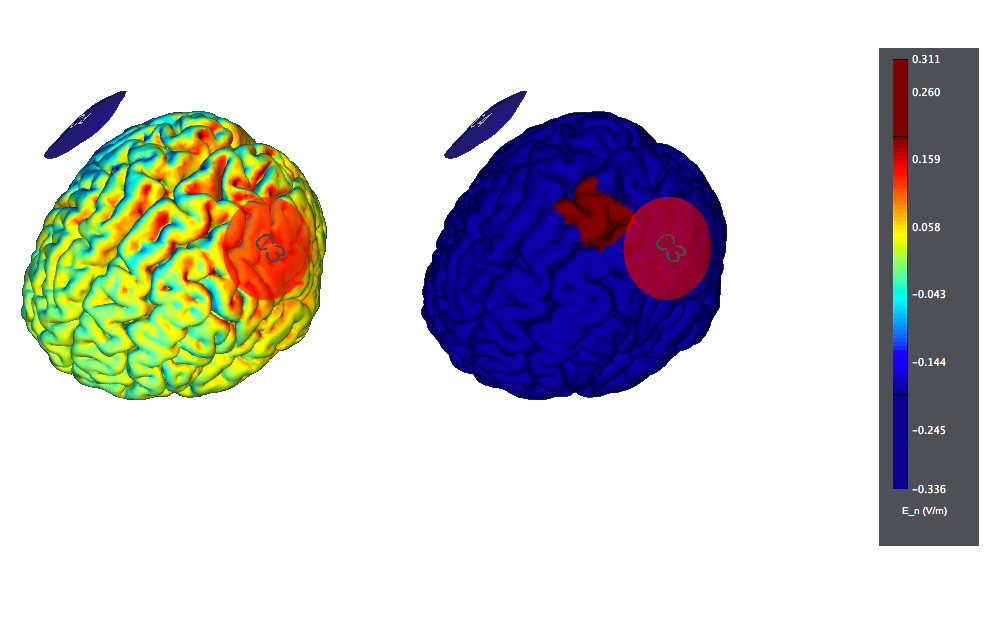
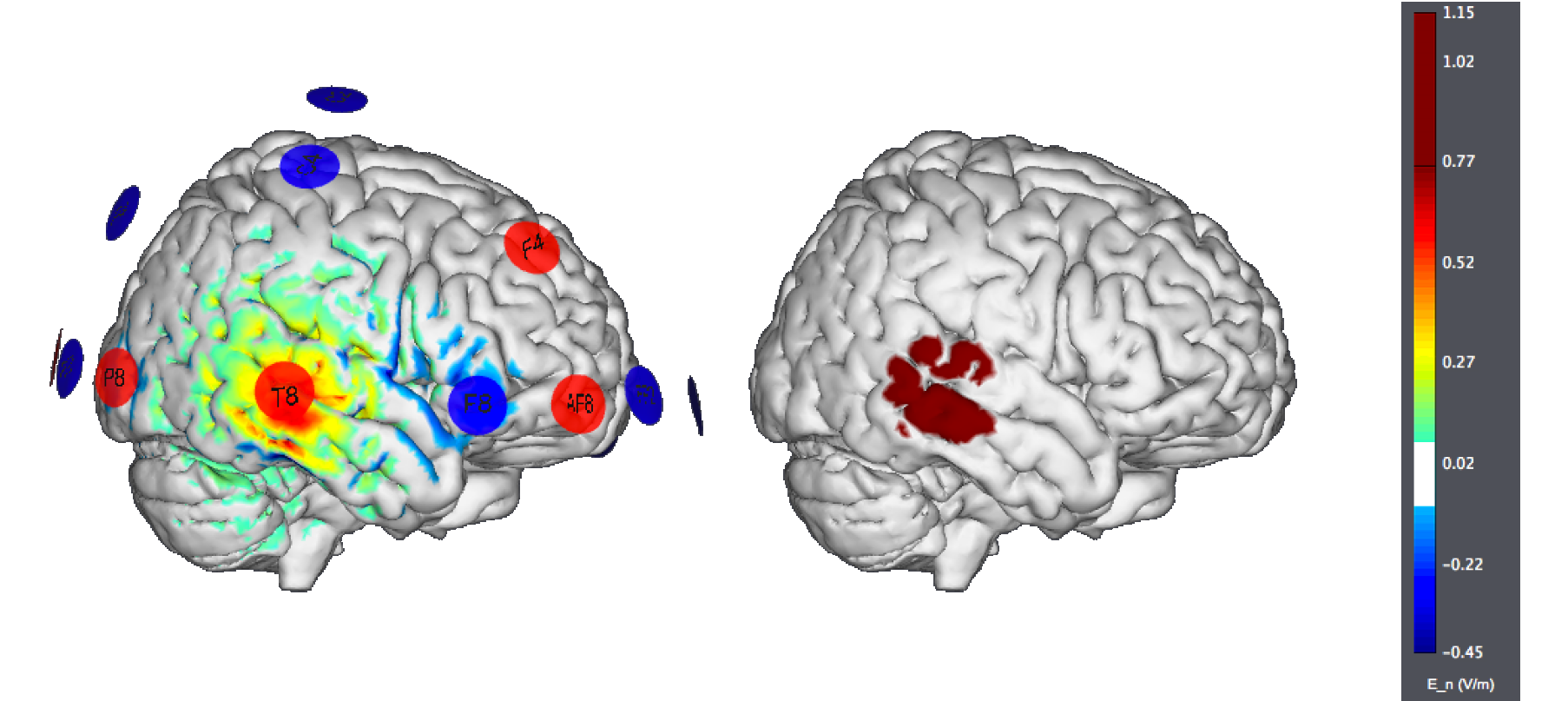
A typical montage using large, traditional sponges will result in large affected areas by the stimulation. It would look like this (the figure on the left displays the normal component of the electric field, the one on the right the target):
An alternative is to put the "reference" electrode over the "contralateral supraorbital region". Again, we see large effects over widespread areas of the cortex:
A more modern version, using Starstim (optimized with StimWeaver) and targeting only the left hemisphere (i.e., trying to avoid stimulating other sites) may look like this:
Pain
For a NEs White Paper on a literature review related to tCS in Pain, please see our recent White Paper <ref > G. Ruffini, tDCS clinical research - highlights: Pain, NE White Paper NEWP201301, 2013 </ref>.
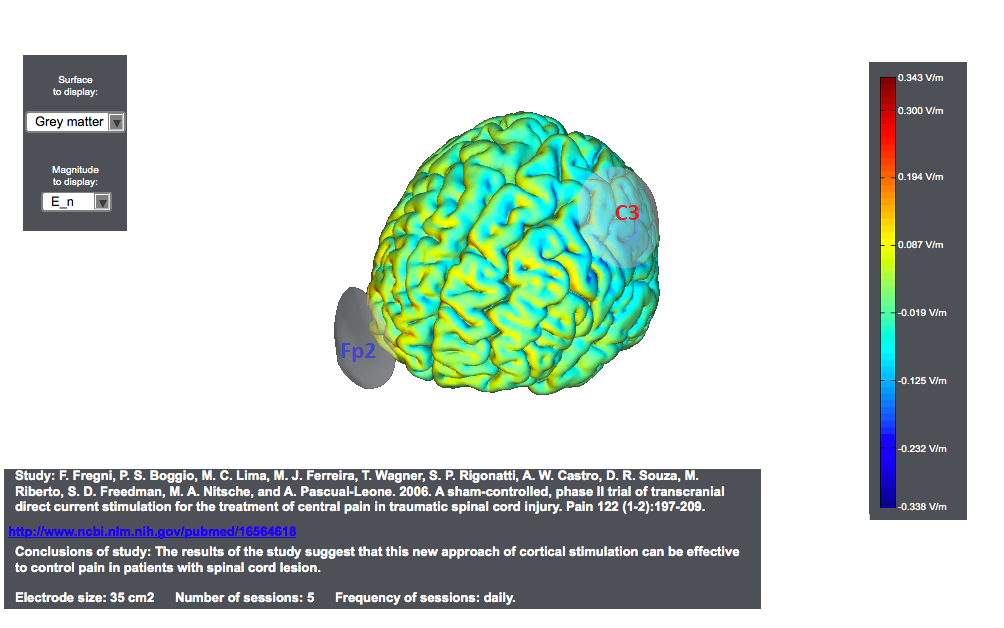
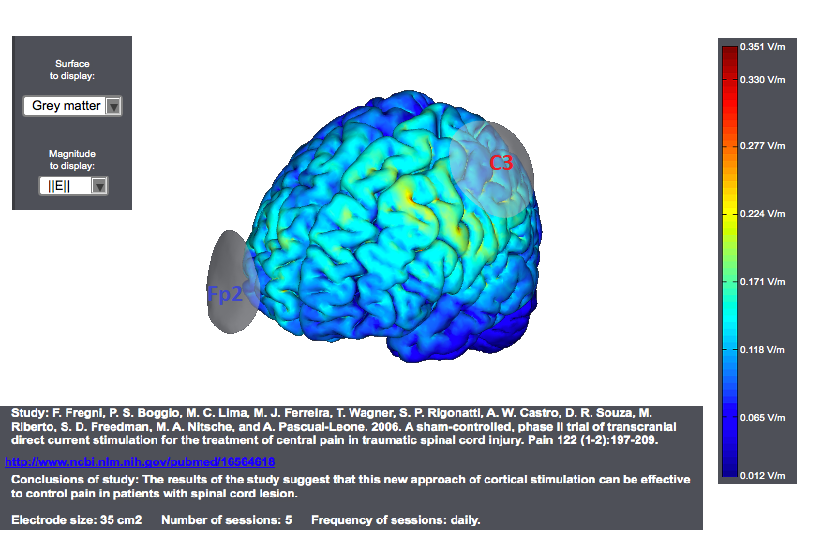
Typical tDCS montages for Pain focus on the primary motor cortex. A montage for central pain from an interesting study<ref> Study: F. Fregni, P. S. Boggio, M. C. Lima, M. J. Ferreira, T. Wagner, S. P. Rigonatti, A. W. Castro, D. R. Souza, M. Riberto, S. D. Freedman, M. A. Nitsche, and A. Pascual-Leone. 2006. | A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 122 (1-2):197-209. </ref> is as follows. The target was the motor cortex, and it relied on the use of large sponge electrodes. The "nuissance" electrode was place on the contralateral orbit. Here we provide a visualization of these montages using StimPreview.
In this study the authors found that there was a significant pain improvement after active anodal stimulation of the motor cortex, but not after sham stimulation. These results were not confounded by depression or anxiety changes. Furthermore, cognitive performance was not significantly changed throughout the trial in both treatment groups. The results of our study suggest that this new approach of cortical stimulation can be effective to control pain in patients with spinal cord lesion.
In most studies, anodal stimulation was applied to M1 of the hemisphere contralateral to pain (in case of focal or lateralized pain) or the dominant (left) hemisphere (in case of more diffuse pain). M1 was usually defined as the location of the C3/C4 electrode in the International 10-20 system for EEG electrode placement.
It has been suggested that M1 anodal stimulation may reduce pain by activating various neural circuits present in the precentral gyrus, which would be afferents or efferents that connect structures involved in sensory or emotional component of pain processing, such as the thalamus or the DLPFC, or by facilitating descending pain inhibitory controls (Lefaucheur, 2006; Nguyen et al., 2011).
The most commonly used protocol consisted of 20-min anodal stimulation of M1 for five consecutive days, which may lead to significant analgesic after-effects lasting for 2 to 6 weeks (Fregni et al., 2006e; Valle et al., 2009; Antal et al., 2010; Kim et al., 2013).
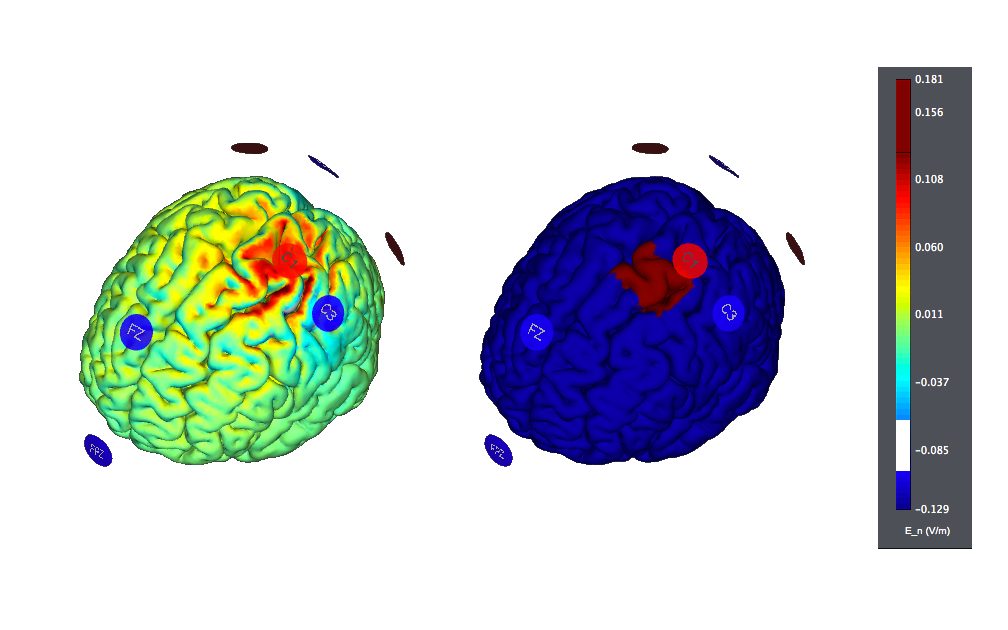
As was the case in Stroke, the use of small electrodes could provide much more focal, controlled stimulation. Here is the 8 ch motor cortex target example derived from StimWeaver (the figure on the left displays the normal component of the electric field, the one on the right the target):
Depression
Please see our recent White paper for more information on tDCS and the treatment of depression<ref > G. Ruffini, tDCS clinical research - highlights: Depression, NE White Paper NEWP201303, 2013 </ref>.
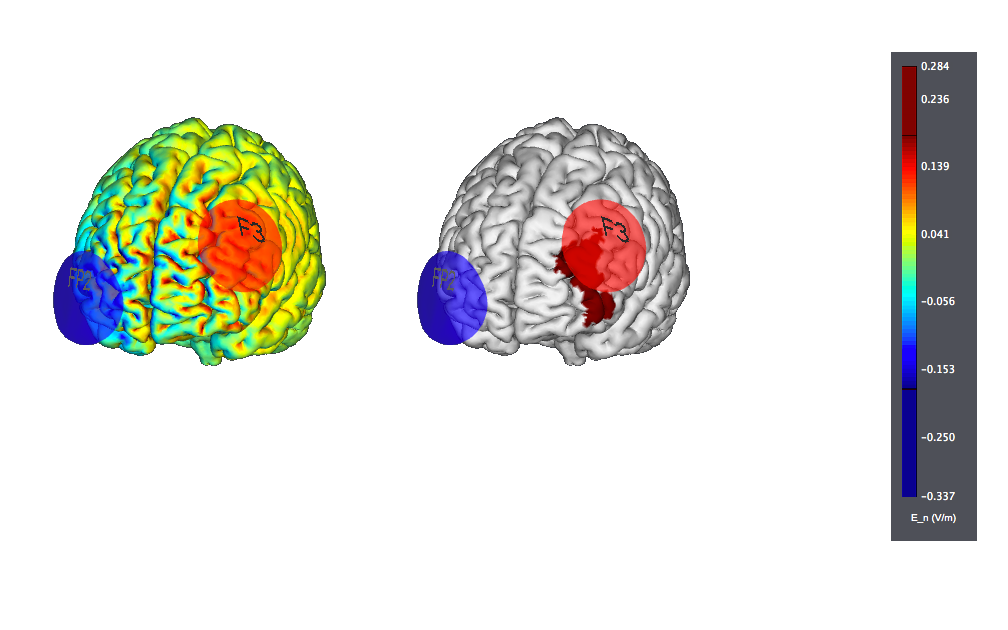
The typical target for treatment is anodal on the left DLPFC (F3) with the cathode over the contralateral orbit or, sometimes, over the right DLPFC.
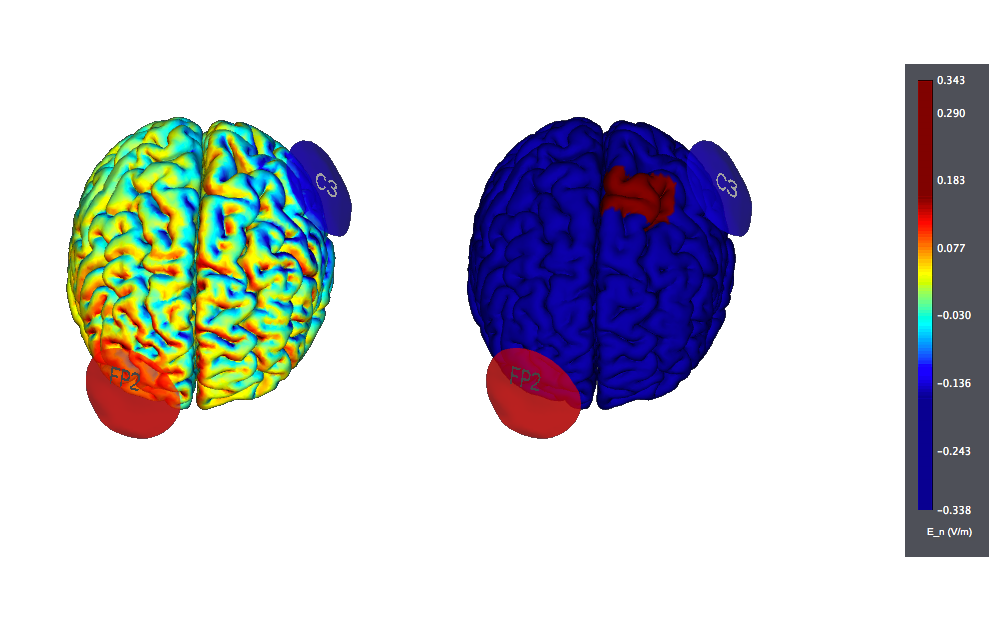
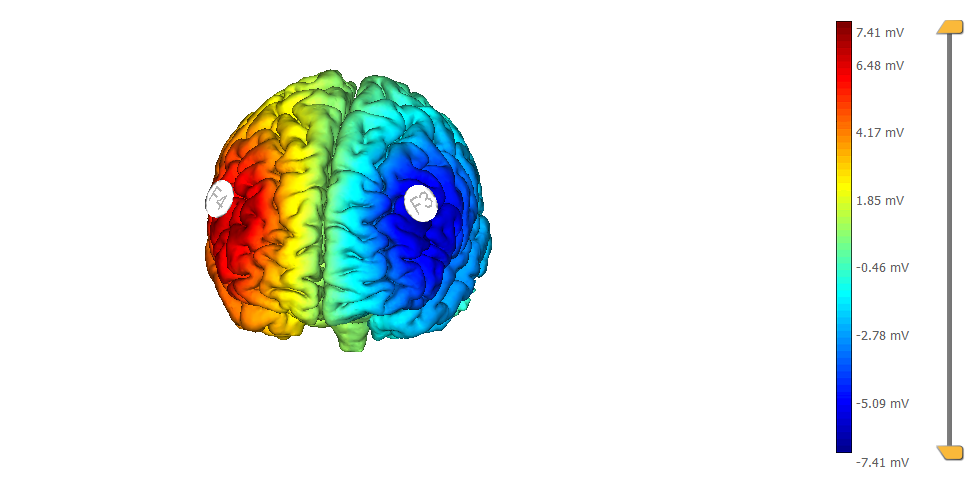
This is a typical tDCS montage: F3 anode vs contralateral orbit (Fp2) using 25 cm2 sponge electrodes. We show also the target, highlighted as left |BA46 (the figure on the left displays the normal component of the electric field, the one on the right the target):
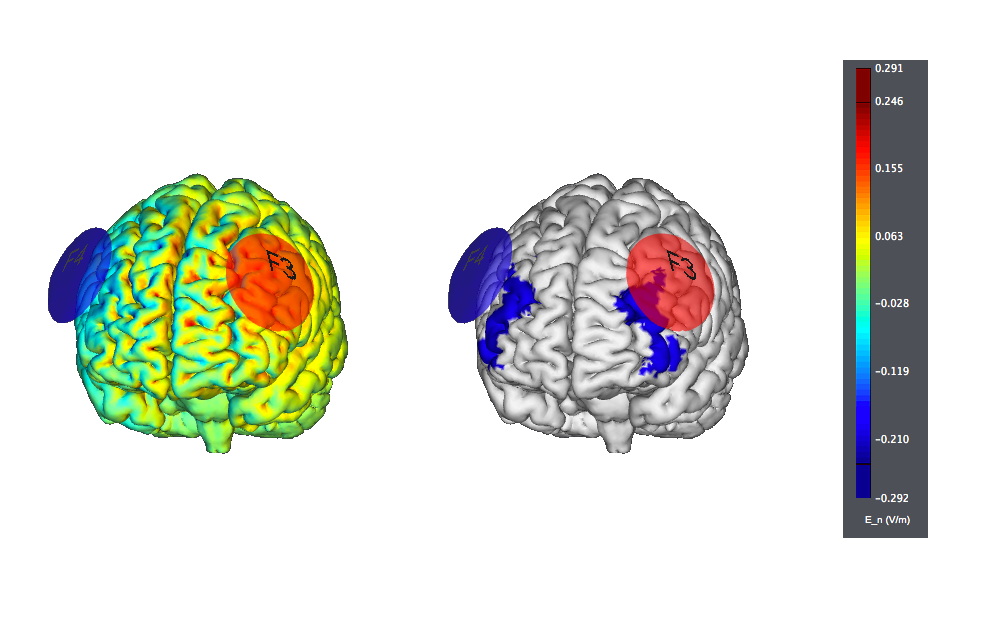
This is another typical montage using sponge electrodes: F3 anode vs F4 orbit. We show also the target, highlighted as left |BA46:
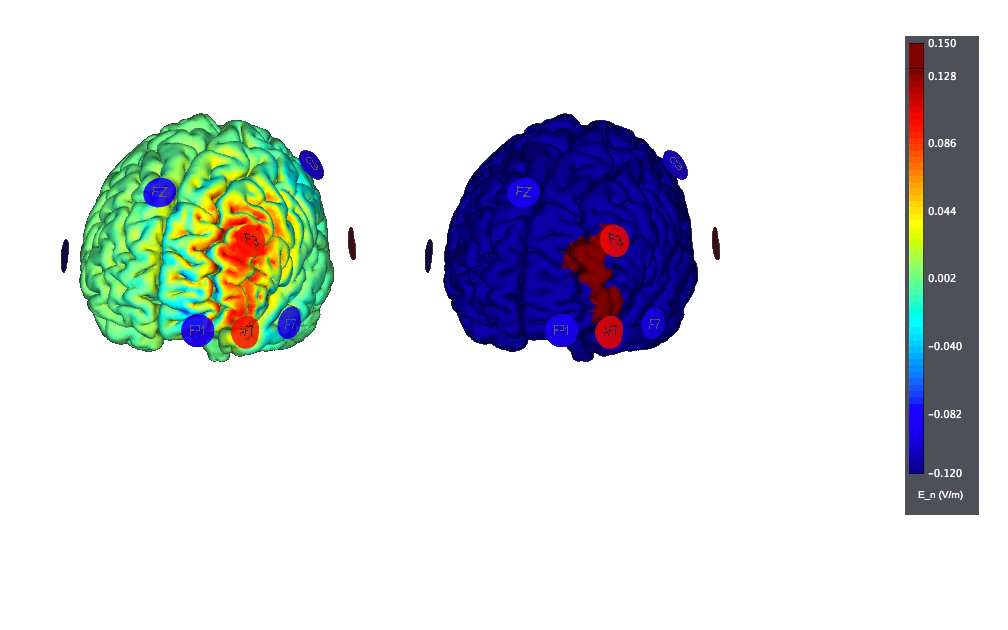
This is a montage optimized using StimWeaver and using 8 Pi-electrodes with StarStim. We show also the target, highlighted as left |BA46. Note the improved focality w.r.t. the target (the figure on the left displays the normal component of the electric field, the one on the right the target):
Tinnitus
Bifrontal transcranial direct current stimulation (tDCS), with the anodal electrode overlying the right and the cathodal electrode overlying the left dorsolateral prefrontal cortex, has been shown to suppress tinnitus <ref> Vanneste S, De Ridder D.,
Bifrontal transcranial direct current stimulation modulates tinnitus intensity and tinnitus-distress-related brain activity.. Eur J Neurosci. 2011 Aug;34(4):605-14.
</ref> <ref> De Ridder D, Vanneste S.
EEG Driven tDCS Versus Bifrontal tDCS for Tinnitus.. Front Psychiatry. 2012 Sep 25;3:84.
</ref> <ref> De Ridder D, Vanneste S.
Bilateral dorsolateral prefrontal cortex modulation for tinnitus by transcranial direct current stimulation: a preliminary clinical study. Exp Brain Res. 2010 May;202(4):779-85.
</ref> <ref> Jae-Jin Song, Sven Vanneste, Paul Van de Heyning, and Dirk De Ridder
Transcranial Direct Current Stimulation in Tinnitus Patients: A Systemic Review and Meta-Analysis. The Scientific World Journal, vol. 2012, Article ID 427941, 7 pages, 2012.
</ref> . Such montages - but with the opposite polarity - have also been used for Depression.
Epilepsy
In general terms anodal tDCS stimulation increases excitability of the cerebral cortex, while cathodal stimulation decreases excitability. Pharmacological therapeutics of epilepsy searches a decrease of excitability to reduce paroxysmal brain cortex activity. From this point of view, the use of multi-electrode montages is particularly appealing, as it allows for controlled stimulation over a particular area with little effects on others.
E.g., using StarStim and targeting only the left motor hemisphere (8 Channels) (i.e., trying to avoid stimulating other sites) may look like this:
and another target problem on the left hemisphere, using 27 Channels, like this (E field on left, target on the right):
Both of these have been produced using the StimWeaver engine.
A study in freely moving rats in a focal epilepsy model showed that cathodal but not anodal tDCS brain stimulation produced a reduction of cortical excitability that could outlasted the actual stimulation in a dependent current strength and duration of stimulation <ref> Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, Löscher W, Paulus W, Tergau F.,
Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy.. Epilepsia. 2006 Jul;47(7):1216-24.
</ref>.
Two clinical trials, one already finished (National Institute of Neurological Disorders and Stroke-NINDS) and the other in course (Spaulding Rehabilitation Hospital) explored the clinical efficacy of the tDSC in human epilepsy. The NINDS protocol study “the effects of repeated applications of tDCS on the excitability of the seizure focus in patients with poorly controlled pharmacologically refractory temporal lobe epilepsy”. The Spaulding study investigated “the feasibility of a closed-loop electroencephalography (EEG) / transcranial direct current stimulation (tDCS) system for treatment of epilepsy. They are looking to see the feasibility of triggering tDCS stimulation within 10 seconds of a detected EEG partial-onset seizure, and also a proof-of-principle determination of whether tDCS applied during this vulnerable period may be feasible to prevent the oncoming seizure”.
Related to use of tDCS in children with epilepsy, a pilot communication <ref> Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, Löscher W, Paulus W, Tergau F.,
Transcranial direct current stimulation for treatment-refractory epilepsy in children: a pilot study. Clinical Neurophysiology, Volume 122, Supplement 1 , Page S2, June 2011.
</ref> reported that cathodal stimulation over the epileptic focus produce a >50 % reduction in seizure frequency in 30 % -assessed with a seizure diary- of 71 pediatric patients with focal or generalized treatment- refractory epilepsy. These reported basic concepts and preliminary results support a potential role for cathodal brain stimulation in the therapeutics of the epilepsy. However this field is just in its first exploratory steps.
Migraine
Generally, the tDCS target is M1, the left dorsolateral prefrontal cortex (DLPFC), or the primary visual cortex (V1) for migraine.
Results suggest <ref> Antal A, Kriener N, Lang N, Boros K, Paulus W., Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine., Cephalalgia. 2011 May;31(7):820-8 </ref> that the application of cathodal stimulation over the V1 might be an effective prophylactic therapy in migraine, at least with regard to pain control. It is thought to decrease its oversensitivity or increased responsiveness at the origin of headache.
In another study, the anode electrode (5 cm x 7 cm) was placed over the motor cortex (contralateral to the most [or predominant] painful side or the side where the symptoms begin) and the cathode electrode (5 cm x 7 cm) was placed over the contralateral supraorbital area. <ref> Dasilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, Spierings EL, Bajwa Z, Datta A, Bikson M, Fregni F., tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine, Headache. 2012 Sep;52(8):1283-95 </ref>
Addictive disorders
A typical montage is prefrontal transcranial direct current stimulation (tDCS) (anode over the right prefrontal cortex and cathode over the left prefrontal cortex). <ref> Rachel L. Goldman, Jeffrey J. Borckard, Heather A. Frohman, Patrick M. O’Neil, Alok Madan, Laura K. Campbell, Amanda Budak, Mark S. George, Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving, Appetite, Volume 56, Issue 3, June 2011, Pages 741–746 </ref> <ref> Victoria C. Wing, Mera S. Barr, Caroline E. Wass, Nir Lipsman, Andres M. Lozano, Zafiris J. Daskalakis, Tony P. George, Brain Stimulation Methods to Treat Tobacco Addiction , Brain Stimulation, Volume 6, Issue 3, May 2013, Pages 221–230 </ref>
Cognitive enhancement
Several locations have been explored, including the bilateral dorsolateral prefrontal cortex (DLPFC) using tRNS <ref> Albert Snowball, Ilias Tachtsidis, Tudor Popescu, Jacqueline Thompson, Margarete Delazer, Laura Zamarian, Tingting Zhu, and Roi Cohen Kadosh, [http://cohenkadosh.psy.ox.ac.uk/publications/articlereference.2013-05-02.2989056797 Long-Term Enhancement of Brain Function and Cognition Using Cognitive Training and Brain Stimulation], Current Biology, 23:987-992. </ref>, the inferior frontal gyrus (IFG) and inferior parietal cortex (IPC) <ref> Sehm B, Schnitzler T, Obleser J, Groba A, Ragert P, Villringer A, Obrig H., Facilitation of inferior frontal cortex by transcranial direct current stimulation induces perceptual learning of severely degraded speech., J Neurosci. 2013 Oct 2;33(40) </ref> and the Posterior Parietal Cortex (PPC) <ref> Tobias U. Hauser, Stephanie Rotzer, Roland H. Grabner, Susan Mérillat and Lutz Jancke, Enhancing performance in numerical magnitude processing and mental arithmetic using transcranial Direct Current Stimulation (tDCS) , Front. Hum. Neurosci., 06 June 2013 </ref>
See our White Paper on Cognitive Enhancement, NEWP-201305 <ref> G. Ruffini, tDCS clinical research - highlights: Cognitive Enhancement, Neuroelectrics White Paper NEWP201305, Oct 2013 </ref> for more information.
References
<references />